Introduction
The outbreak of the COVID-19 pandemic, an unprecedented black swan event has forced economies across the world to reassess their preparedness, capabilities and infrastructure for a health crisis of this magnitude. Recently, it was also reported that India reached nearly two million Covid-19 cases in the month of August’20, setting the highest monthly tally in the world since the pandemic began1. As of September 24, 2020, the number of global active cases has reached nearly 31.9 million, with the total death count of 977,000. India, on the same day, reported over 57 lakh cases and 91,149 fatalities2.
With the number of cases growing exponentially across the country, the pressure on the Indian healthcare infrastructure is mounting. In the absence of a vaccine, severe shortage of beds and the sheer population of the country, the need for affordable and easy-to-use ventilators has become ever so important.
Today, India is facing a conspicuous shortage of affordable ventilators. While the government is encouraging indigenous manufacturers to come forward, there still is a disproportionate reliance on specialised, proprietary, and mass-manufactured resuscitators from a small selection of suppliers. Moreover, of the 48,0003 ventilators available in India, 93%4 are concentrated among a few states alone: Uttar Pradesh, Karnataka, Maharashtra, Tamil Nadu, West Bengal, Telangana and Kerala considering the large number of cases being reported.
A cost-effective solution for India, AQovent™ is sure to contribute significantly to the nation’s fight against the pandemic
Aravind Melligeri
Chairman, Aequs

The effort to combat the health crisis has exposed the weak links in the component production ecosystem for medical equipment in India. The key challenges faced are of achieving scale, quality and speed to market, resulting in a continued reliance on imports.
Addressing the need gap
The endeavour to address the need gap of affordable ventilators in the Indian healthcare infrastructure has been initiated from several quarters from the top down. One of these initiatives is the PM Cares fund, which has allotted INR 2000 crores to purchase 50,000 Made-in-India ventilators. The other notable effort is the Drugs Controller General of India’s open call as on March 23, 2020, encouraging all companies across the country to manufacture affordable ventilators/resuscitators.
Timely innovation
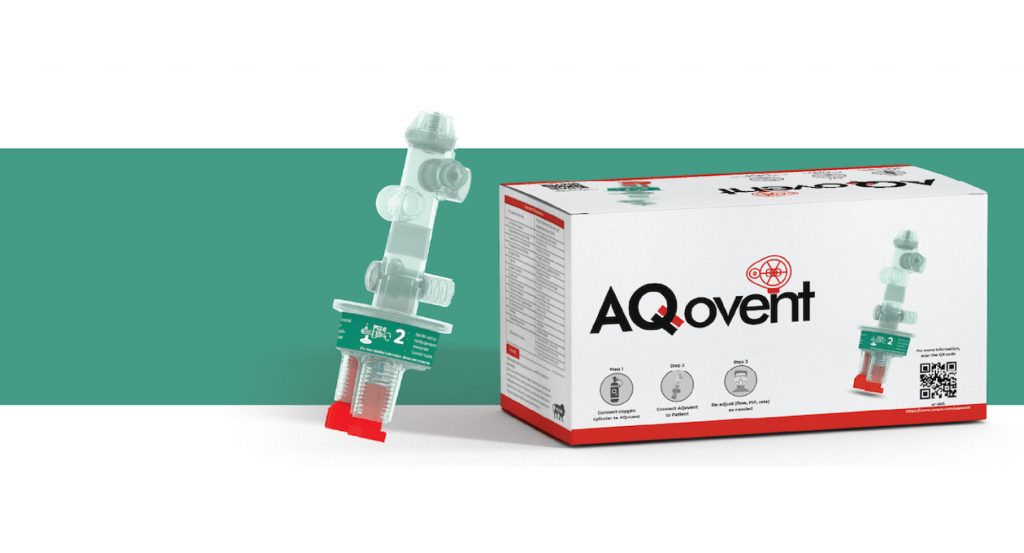
In line with this, Aequs has stepped up to leverage Aerospace and Consumer Divisions’ collective capabilities in its vertically integrated ecosystem at the Special Economic Zone in Belagavi, Karnataka manufacture AQovent™, a medical-grade, low-cost, mass-produced mechanical resuscitator.
As one of the leading manufacturing partners of global OEMs like Airbus, Boeing, Collins Aerospace, SAFRAN, GKN Aerospace, and others, Aequs specializes in high-level precision engineering and ensures delivery of ‘zero-defect’ product quality. This inbuilt potential has been leveraged ably in the manufacturing of AQovent™, making it superior and safe to use.
The driving force for this innovation has emerged from our commitment towards the community and solidarity towards the nation’s effort to fight the pandemic.
The AQovent™ Journey
- The Journey of the project began with an invitation from University of Illinois, U.S.A. to adopt their concept design for the resuscitator. The consumer division decided to pursue this invitation and initiated the licensing process and completed the agreement within three days to kick-start the project.
- Over the course of product development, through value engineering, advance design changes were brought into the product while keeping intact its functional integrity, to enhance ease of manufacturing and reduce the lead time to market, thereby making it conducive for mass production. A team of cross-functional engineers from our aerospace and consumer divisions pooled in their collective expertise to achieve the requisite design parameters, following which, a 3D prototype of the resuscitator was printed to assess the product functioning criteria, in order to accelerate product development. The moulds for AQovent™ were developed in-house using the Aerospace CNC machines, and subsequently produced by our consumer division. The manufacturing process was carried out in three phases: Moulding of the parts; assembly of Patient Tee, Manometer and Modulator using the concept of Taper Fit for ease of assembling; and testing at every stage for functional validation and safety measurement.
- These mechanical resuscitators have been qualified and tested for functional design. The testing was performed at every stage of development and product assembly, in multiple cycles, under varying conditions. The tests performed under the pre-clinical and clinical trials include endurance, performance, and product calibration testing. Additionally, the primary packaging of AQovent™ is also put through a sterilisation process. Unit-wise test results were recorded throughout the process to analyse the consistency of its functional parameters and traceability.
- Uniquely designed to suit the Indian conditions, these resuscitators can be operated entirely on oxygen supply, thereby enabling them to be used in non-electrified regions and in situations where there is a limitation of medical infrastructure. Moreover, its portability makes it usable in ambulances as well. Born of cutting-edge technology, the resuscitators are compact and easily deployable, providing flexibility for treating patients, who are not only affected by the virus, but also by other respiratory conditions.
- The AQovent™ project is an initiative of Aequs Foundation, Aequs’ CSR arm and is manufactured by its Consumer division. This is a non-profit undertaking, and we have committed to deliver these resuscitators initially to Tier-II and Tier-III cities in the state of Karnataka. We are looking at a phased distribution of the product to government hospitals, health centres, and NGOs in these markets.
“A cost-effective solution for India, AQovent™ is sure to contribute significantly to the nation’s fight against the pandemic and will go a long way in democratizing the response to the crisis. Its affordability and scalability are intended to meet the surge in demand for ventilators in the country. This initiative is a testament to Aequs’ commitment to the society, the nation, and India’s ‘COVID-warriors'”, Aravind Melligeri, CEO & Chairman, Aequs Inc.
1https://www.republicworld.com/world-news/us-news/who-reports-highest-spike-in-cases-across-the-globe.html
2https://www.outlookindia.com/website/story/india-news-covid-19-update-india-cross-57-lakh-cases/360831
3This is the official report available on the relevant statistics on ventilators. The numbers remain unchanged-https://cddep.org/wp-content/uploads/2020/04/State-wise-estimates-of-current-beds-and-ventilators_20Apr2020.pdf
4Figure calculated from the proposal shared by Aequs.